
By Riko Umeda
Ammonia, also known as NH3, is an extremely important substance for agriculture because it is a major component used in fertiliser for crops. However, currently, 80% of ammonia production results in huge amounts of greenhouse gases. This is clearly a problem given current concerns about the environment. In a recent study, though, Kyuha Lee and his team found a potential solution that will decrease the amount of greenhouse gases entering the atmosphere every time ammonia is produced.
How is Ammonia Produced Today?
The Haber process is the most commonly used process in the production of ammonia today and was developed at the beginning of the 20th century. In this process, hydrogen and nitrogen are synthesised to form ammonia.
3H2 + N2 → 2NH3
The way hydrogen is extracted during the process renders the method problematic. Currently, hydrogen used in the Haber process is extracted from natural gas, which causes greenhouse gas emissions as shown in the equations below. This process is called natural gas-based ammonia production.
CH4 + H2O → CO + 3H2
CO + H2O → CO2 + H2
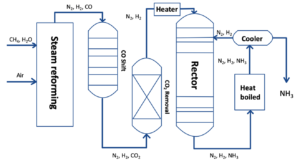
Figure 1 Haber Process
Currently, the large amount of CO2 that is produced from this process, alongside the fossil fuels used to maintain it, is not healthy for the environment. To address this problem, Lee and his team investigated two new processes that look promising for reducing the amount of greenhouse gases that are released into the atmosphere every time ammonia is produced.
Method 1
The first method captures any extra CO2 that is produced when making ammonia. In this process, the production of ammonia follows the same pattern as the conventional ammonia-producing cycle. What is unique about this process, however, is that this method captures the CO2 produced when hydrogen is extracted and transported by pipelines for storage or utilisation. This method can reduce 55–70% of greenhouse gas emissions to the atmosphere compared to the conventional ammonia production method. The liquid produced results in excess heat, which can be converted to produce steam. Then, the steam can be used to turn a turbine, thereby generating electricity, which can be used to compress nitrogen and hydrogen together as shown in the first equation. Lee and his team suggest a unique engineering model that generates electricity by turning turbines. This is a huge advancement as it means that overall the net electricity requirements are lower and there is a lesser dependence on fossil fuels for electricity.
More work needs to be done to ensure all of this can be put into action without the inhibiting costs that often come with advances in green chemistry. For example, transporting gases used in this process requires a lot of energy as the temperature and pressure in the pipelines need to be kept at 50 degrees and 2200 pound per square inch. Therefore, there are some issues with this approach, such as the cost to maintain these conditions in the pipelines. As the overall price would be more expensive, this process is not necessarily cost-competitive with the natural gas-based ammonia production method, even though it is ultimately better for the environment.
Method 2
In a second method, Lee and his team suggest a method that completely eliminates greenhouse gases altogether. Ammonia is produced through a combination of water electrolysis, air separation, and the Haber process. As a lot of scientists are keen on developing a decarbonised society, there are methods that synthesise ammonia from carbon-free sources such as water, which is uxsed to extract hydrogen in electrolysis. In addition, the electricity used in these methods is zero carbon or near-zero carbon as it is generated from nuclear, solar, and wind power. Therefore, the greenhouse gas emission is very close to zero. The problem is the expense. Lee and his team suggest that to be cost-competitive with other methods of ammonia production, the cost of hydrogen needs to be cheaper. The cost of hydrogen in this method is expensive because first, water electrolysis – which is an energy-intensive process – is used, while second, the electricity utilised in electrolysis often comes from nuclear power or renewable resources, which is more expensive than fossil fuel energy.
Overall, these researchers found that both methods were better than the natural gas-based ammonia production from the perspective of greener chemistry. Nuclear and renewable ammonia production methods can reduce huge amounts of greenhouse gas emissions compared to carbon-capturing ammonia production. However, these methods are about three times more expensive than the traditional method that captures CO2. The difference between carbon-capturing methods and nuclear, renewable ones is that in the carbon-capturing methods, the cost required to treat CO2 makes this process expensive. On the other hand, in nuclear and renewable ammonia production, the cost to extract hydrogen by water electrolysis is expensive. Although scientists have now found two solutions for cutting down on greenhouse gas emissions, more research is needed to find better ways of cutting down on the costs required to ensure these processes can be both cost-competitive and help the environment in today’s market.